activation energy graph

The graph above shows how the activation energy is lowered in the presence 
be visualized as a barrier that must be overcome by reactants before 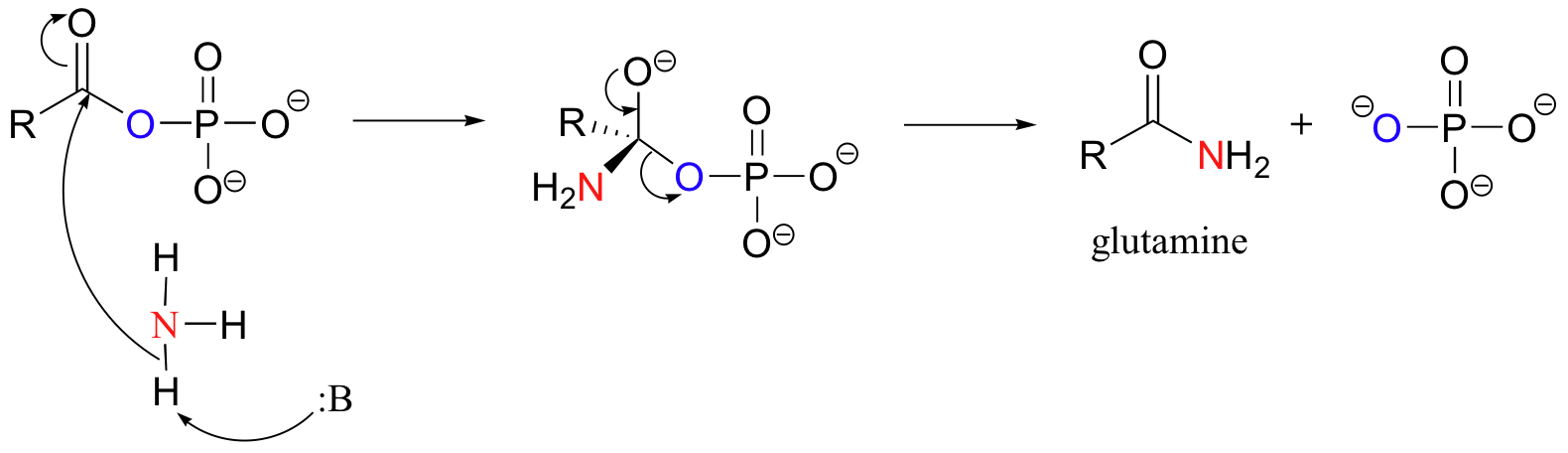
Left graph shows a positive overall activation energy (Ea > 0) and right 
Activation Energy meaning more particles now have the required energy 
the activation energy and the relaxation time of the process. Fig.
The uphill portion of the graph represents 
This graph shows how the presence of an enzyme lowers the activation energy 
In other words, to move the activation energy on the graph like this:
times by providing reaction route having a smaller activation energy:
With this graph, which looks something like this, we will be able to see 
From the above data, the following graph is drawn, and the slope is = -13622
means fewer or more particles will have the required activation energy.
Therefore, if the reaction goes from higher free energy to lower free energy 
An activation energy diagram for an endothermic reaction is illustrated here 
diagram from the preceding question, what is the activation energy for 
A catalyst may Activation energy graph work by lowering the activation energy for a reaction.Physics Question: Which Pink Line On The Graph Below Represents
On this graph, which letter represents the activation energy?
The graph represents the basis of enzyme reactions – lowering the activation 
to go over an energy "hill" called the "energy of activation" to occur.
Frequency factor, k0 = 1.25 s-1; Activation energy, Ea = 380 J/mol;




















Currently have 0 comments: